By Gabriella Borter and Michael Erman
Tristen Sweeten, a 34-year-old nurse in Utah, hopes her three children will receive Moderna’s COVID-19 vaccine through its pediatric clinical trial. The sooner the better, she said, for their safety and the greater goal of ending the pandemic.
Angie Ankoma, a 45-year-old Black mother of four who works in philanthropy in Rhode Island, believes trials must include diverse populations and participated in one for a COVID-19 vaccine herself. Volunteering her kids for possible inclusion in Moderna’s trial was a tougher call.
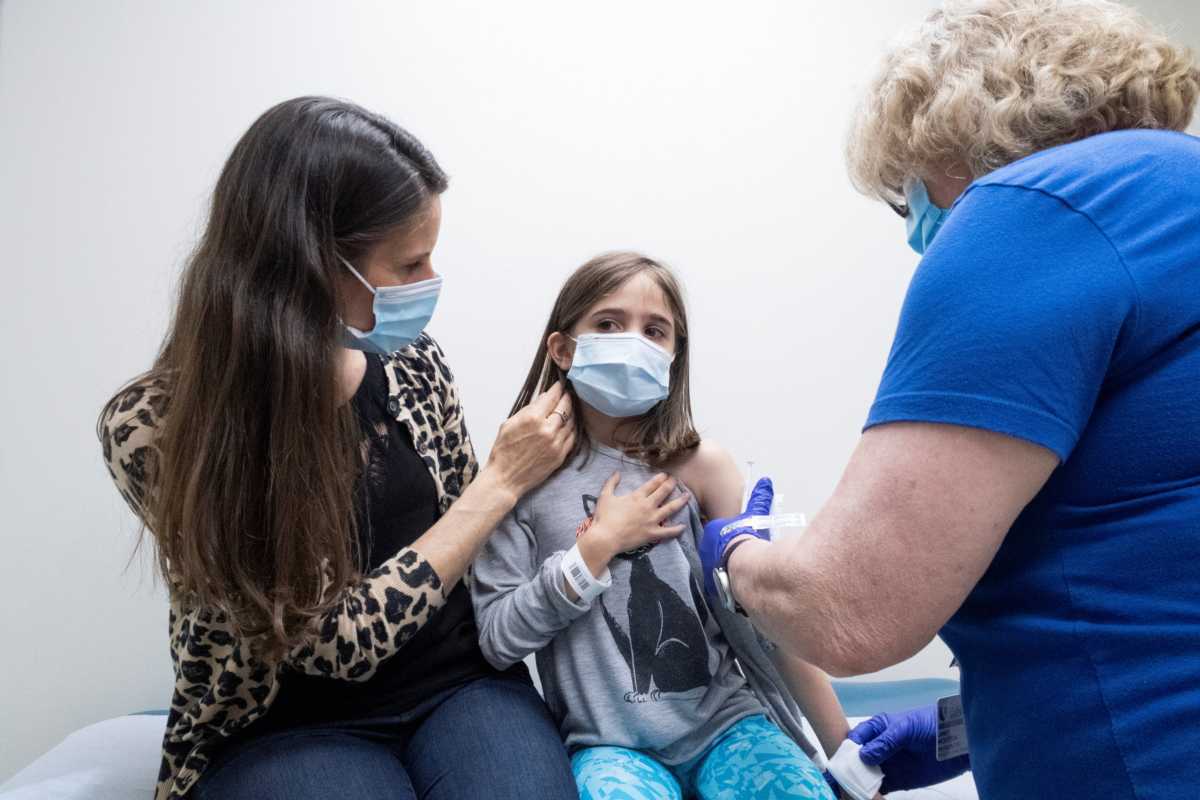
Sweeten and Ankoma are among thousands of U.S. parents who volunteered to have their children participate in new trials run by Pfizer with BioNTech or Moderna, the first companies making strides toward developing a safe COVID-19 vaccine for the country’s 48 million children under age 12.
Health officials say vaccines are crucial to ending the pandemic. But many are concerned vaccine hesitancy in some adults will be even more pronounced when it comes to their children. Parents may question the risks versus benefits, given the unknowns about the vaccines’ long-term impact on children’s’ development and data on how few young kids have been hit hard by COVID-19.
To ease those concerns, some scientists say the U.S. Food and Drug Administration should slow the review process for pediatric COVID-19 vaccines.
Pfizer spokeswoman Jerica Pitts said it was premature to speculate on an approval pathway for children, but the company plans to work with public health institutions to promote the importance of vaccines.
Moderna research scientist Dr. Jacqueline Miller said the company has talked to the FDA about the best way to clear the vaccine for use in kids. She said the company hopes to make the vaccine available to children through emergency use authorization (EUA) that got it to U.S. adults in record time, in part to be able to get kids back to school “and the things that they all are longing to be doing.”
Sweeten’s husband Scott is a clinical researcher whose company has worked on the Johnson & Johnson and AstraZeneca adult vaccine trials, so the couple, whose children are ages 5, 8 and 10, are comfortable with how they were developed, Tristen said.
“We feel like they’re very safe,” she said.
Ankoma consulted her pediatrician given her nagging doubts about unknown long-term effects. She ultimately decided the risk was worth it to immunize her four kids, ages 7 to 16.
“It was easier for me to decide for myself than it was for the kids, because…it was my own body,” she said.
Researchers leading pediatric trials for Moderna and Pfizer in children as young as 6 months feel confident the vaccines will be just as safe and effective for children as they have been for adults.
Pfizer’s vaccine, already available to people aged 16 and up in most U.S. states, was found to work well in children 12 to 15 and may receive regulatory authorization for that age group as soon as next month.
Moderna and Pfizer have said vaccines could be widely available to even younger children by early 2022.
A Reuters/Ipsos poll from April 12-16 found that 55% of U.S. parents said they were interested in getting their kids vaccinated.
Children under 12 have so far been at relatively low risk from the coronavirus.
Still, some 284 children have died from COVID-19 since last May, about 0.06% of all COVID-19 deaths, according to American Academy of Pediatrics data from about 43 states. There were 14,500 hospitalizations among children in 24 states during that time, about 2% of the total.
Dr. Sean O’Leary, a pediatrics professor at the University of Colorado, said vaccination will help children avoid hospitalizations, a rare inflammatory reaction or lasting symptoms known as long COVID.
“It is certainly not correct to say it’s benign in children. Anyone that’s worked in a children’s hospital can tell you how many sick kids we’ve taken care of,” he said.
Children already receive vaccines for illnesses that have similar or lower levels of related mortality in kids, like hepatitis A, varicella, rubella and rotavirus.
Health officials warn that if left unvaccinated, children could be a reservoir for infection, allowing virus variants that may evade vaccines to circulate and grow.
That these vaccines will have been widely used in adults before being made available for children should reassure parents, said Emmanuel Walter, head of Pfizer’s pediatric vaccine trial at Duke University.
Some other vaccines have been developed for and only given to children, such as the chicken pox shot.
More than 63 million Americans have received the Pfizer vaccine and about 55 million the Moderna shot.
The trials for young children are more involved than for adolescents because they begin by testing very small doses and gradually increase the dosage while monitoring for side effects.
“What we’re trying to find is that Goldilocks moment when we have just enough vaccine to generate a really good immune response, but we don’t have so much that we’re causing a lot of fever and arm pain and distress in the baby or in the younger child,” said Buddy Creech, a Vanderbilt University professor working on Moderna’s pediatric trial.
Some scientists said waiting for standard approval instead of seeking an EUA would add months to the timetable but allow for gathering more safety data that could help boost public confidence.
The FDA declined to comment.
Dr. Cody Meissner, head of pediatric infectious disease at Tufts University’s medical school, said it comes down to one question: “Does the low burden of disease in children justify a more protracted evaluation of safety?”
Reuters