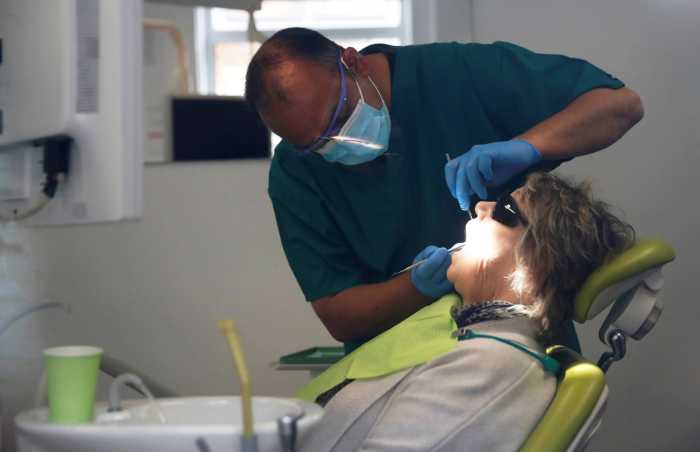
The Trump administration plans emergency approval of a new coronavirus therapeutic treatment and is considering fast-tracking a vaccine developed in Britain, according to media reports on Sunday, the day before the start of the Republican National Convention.
President Donald Trump later on Sunday will announce the emergency authorization of convalescent plasma for COVID-19, a treatment that already has been given to more than 70,000 patients, the Washington Post reported, citing officials familiar with the decision.
The action will be highlighted at a news conference scheduled for 5:30 p.m. EDT (2130 GMT), the newspaper said.
A White House spokesman declined to comment on the Post story. But White House Press Secretary Kayleigh McEnany tweeted early on Sunday that Trump’s news conference would cover “a major therapeutic breakthrough on the China Virus”.
Trump also tweeted: “Important White House News Conference at 5:30 (sharp) today. Very good news!”
The White House also declined comment on a separate report in the Financial Times that the administration is considering fast-tracking an experimental COVID-19 vaccine being developed by AstraZeneca Plc and Oxford University for use in the United States ahead of the Nov. 3 elections.
One option being explored would involve the U.S. Food and Drug Administration (FDA) awarding “emergency use authorization” in October to the potential vaccine, which was developed by Oxford and licensed to AstraZeneca, the FT reported, citing people briefed on the plan.
Trump is looking to boost his lagging poll numbers during the Republican convention this week, and progress in treatments or an effective vaccine to gain control of the virus would aid his re-election chances.
The U.S. Centers for Disease Control and Prevention (CDC) on Sunday said the number of deaths due to the new coronavirus had risen by 1,006 to 175,651. It reported 5,643,812 cases, an increase of 45,265 cases from its previous count.
AstraZeneca denied having discussed an emergency use authorization for its potential vaccine with the U.S. government.
“AstraZeneca has not discussed emergency use authorization with the U.S. government and it would be premature to speculate on that possibility,” a spokeswoman for AstraZeneca said in a statement.
The company said that the late-stage Phase 2 and Phase 3 trials for its vaccine candidate are still ongoing in Britain and other markets globally and that it did not anticipate efficacy results until later this year.